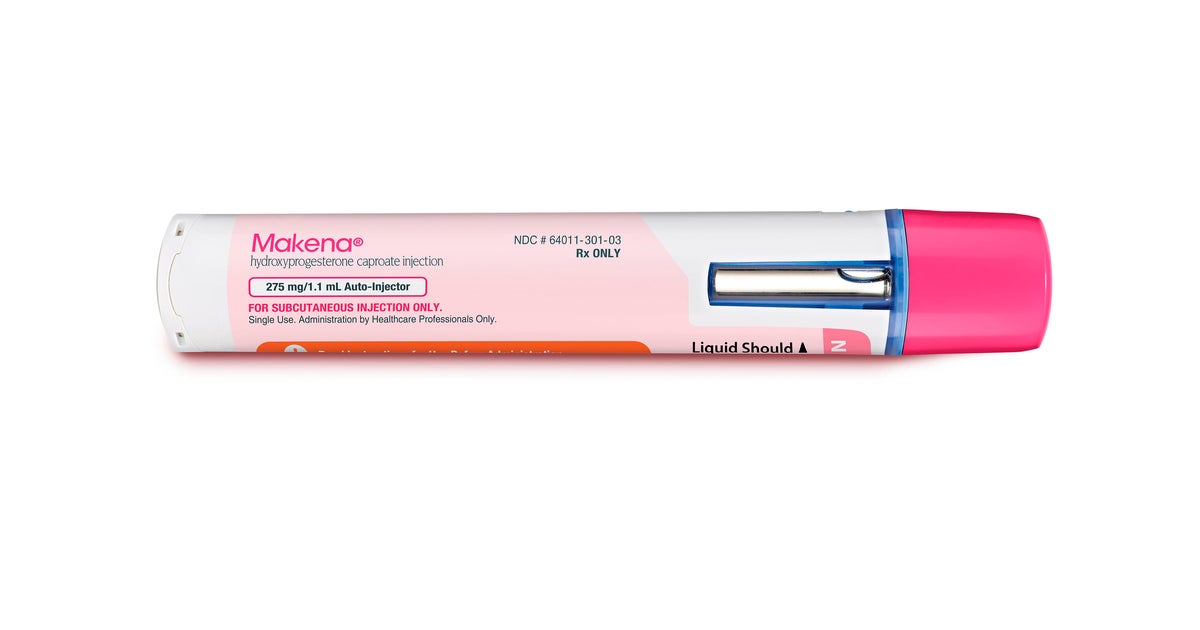
Hence then, the article about fda takes only drug for premature birth off the market was published today ( ) and is available on CBS sacramento ( Middle East ) The editorial team at PressBee has edited and verified it, and it may have been modified, fully republished, or quoted. You can read and follow the updates of this news or article from its original source.
Read More Details
Finally We wish PressBee provided you with enough information of ( FDA takes only drug for premature birth off the market )
Last updated :
Also on site :
- Today’s NYT ‘Strands’ Hints, Spangram and Answers for Saturday, January 17, 2026
- Maison Margiela’s 'Elegant' Duo Perfume Set Makes Fragrance Layering Easy, and It’s Just $30 at Kohl’s
- Royal Caribbean Extends Pause at Private Caribbean Resort Through 2026—Here’s What Cruisers Are Getting Instead